
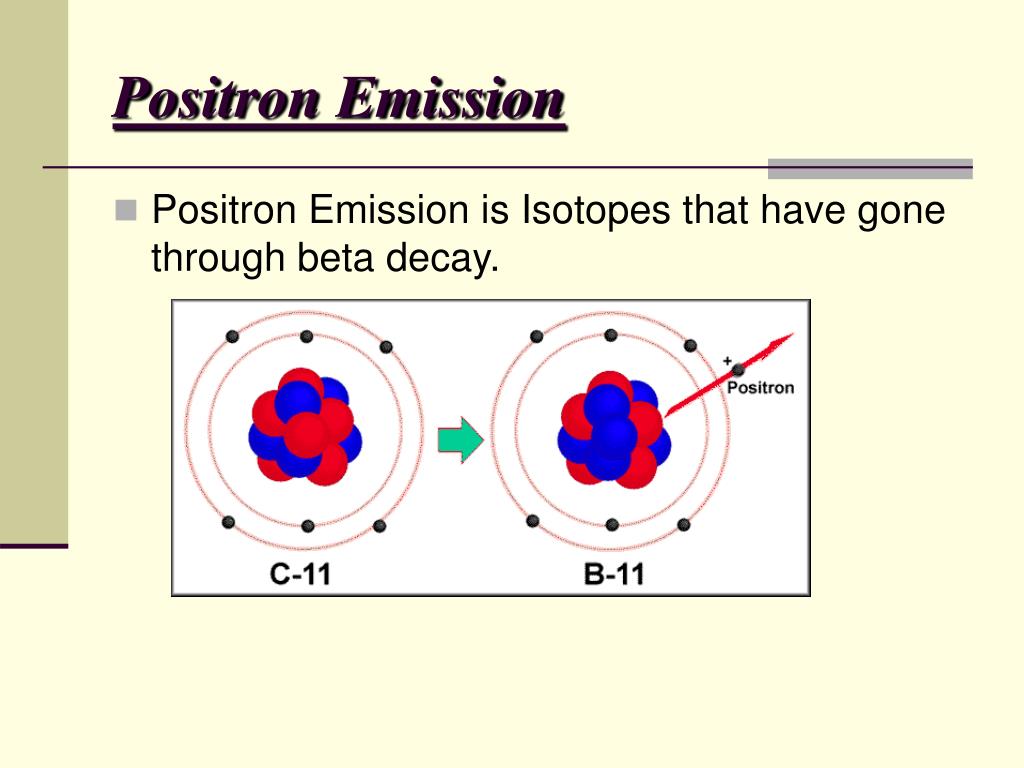
Positron emission is a type of radioactive decay where a proton inside a radioactive nucleus is converted into a neutron while releasing a positron and an electron neutrino. Key Terms: Atom, Electron, Electron Neutrino, Nucleus, Neutron, Positron, Proton, Radioactive Decay

What is the Difference Between Positron Emission and Electron Capture What are the Similarities Between Positron Emission and Electron CaptureĤ. This is the main difference between positron emission and electron capture. In positron emission, a proton inside the radioactive nucleus is converted into a neutron while releasing a positron in electron capture, a proton-rich nucleus of a neutral atom absorbs an inner shell electron which then converts a proton into a neutron, emitting an electron neutrino. Both these processes take place in proton-rich nuclei. Electron capture is a process which emits an electron neutrino. Positron emission is the release of a positron and an electron neutrino in the process of radioactive decay. There are different decay pathways such as positron emission, negatron emission and electron capture. Radioactive decay causes an isotope of a particular element to be converted into an isotope of a different element. Therefore, in order to become stable, these isotopes undergo a spontaneous process called radioactive decay. There are certain naturally occurring isotopes that are unstable due to the imbalanced numbers of protons and neutrons they have in their nucleus of atoms. Main Difference – Positron Emission vs Electron Capture


 0 kommentar(er)
0 kommentar(er)
